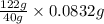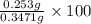An unknown amount of acid can often be determined by adding an excess of base and then back-titrating the excess. a 0.3471−g sample of a mixture of oxalic acid, which has two ionizable protons, and benzoic acid, which has one, is treated with 97.0 ml of 0.1090 m naoh. the excess naoh is titrated with 21.00 ml of 0.2060 m hcl. find the mass % of benzoic acid.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
You know the right answer?
An unknown amount of acid can often be determined by adding an excess of base and then back-titratin...
Questions in other subjects:

Mathematics, 14.01.2020 18:31



Social Studies, 14.01.2020 18:31

History, 14.01.2020 18:31



Mathematics, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31

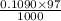
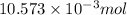
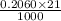
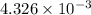 mol
mol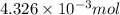
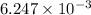 mol
mol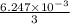
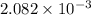 mol
mol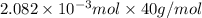
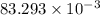 g
g