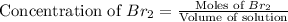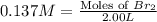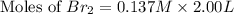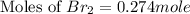Chemistry, 11.10.2019 03:00 simiyi1983
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen bromide: h2 (g) + br2 (g) ↔ 2hbr (g) a mixture of 0.682 mol of h2 and 0.440 mol of br2 is combined in a reaction vessel with a volume of 2.00 l. at equilibrium at 700 k, there are 0.516 mol of h2 present. at equilibrium, there are mol of br2 present in the reaction vessel.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, WhiteWinterRose
What is the chemical formula of the following compound
Answers: 3

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

You know the right answer?
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen...
Questions in other subjects:


Mathematics, 11.11.2020 02:00



Mathematics, 11.11.2020 02:00


Mathematics, 11.11.2020 02:00

Mathematics, 11.11.2020 02:00


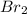 at equilibrium is 0.274 mole.
at equilibrium is 0.274 mole.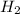 and
and 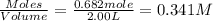
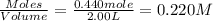
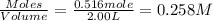
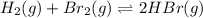
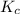 will be,
will be,![K_c=\frac{[HBr]^2}{[H_2][Br_2]}](/tpl/images/0308/9786/3b8cb.png)