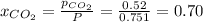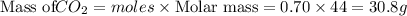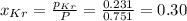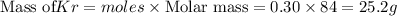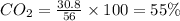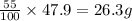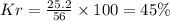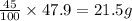Be sure to answer all parts. a mixture of co2 and kr weighs 47.9 g and exerts a pressure of 0.751 atm in its container. since kr is expensive, you wish to recover it from the mixture. after the co2 is completely removed by absorption with naoh(s), the pressure in the container is 0.231 atm. (a) how many grams of co2 were originally present? (b) how many grams of kr can you recover?

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Be sure to answer all parts. a mixture of co2 and kr weighs 47.9 g and exerts a pressure of 0.751 at...
Questions in other subjects:

Mathematics, 15.09.2020 20:01

English, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

History, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

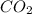 in mixture = 26.3 grams
in mixture = 26.3 grams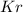 in mixture that can be recovered= 21.5 grams
in mixture that can be recovered= 21.5 grams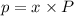
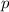 = partial pressure
= partial pressure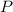 = total pressure = 0.751 atm
= total pressure = 0.751 atm = mole fraction
= mole fraction