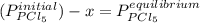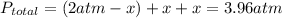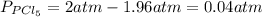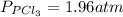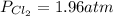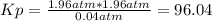Chemistry, 10.10.2019 18:10 lailabirdiemae
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium according to the reaction: pcl5(g) ⇄ pcl3(g) + cl2(g) analysis shows the total pressure in the flask at equilibrium is 3.96 atm. calculate the equilibrium constant kp for the reaction.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium acc...
Questions in other subjects:










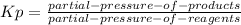
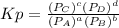
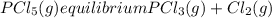
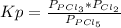
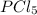 so the total pressure for the system is also the partial pressure for
so the total pressure for the system is also the partial pressure for 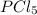 ,
,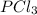 and
and 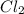 so now we need to estimate the partial pressure for each specie.
so now we need to estimate the partial pressure for each specie. 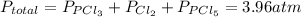
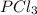 and
and 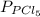 in the equilibrium will be
in the equilibrium will be