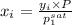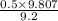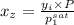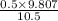Chemistry, 10.10.2019 05:30 aleahnew36
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is initially all liquid at 120°c and a high pressure, and the pressure is gradually reduced at a constant temperature. estimate the pressures at which the first bubble of vapor forms and at which the last drop of liquid evaporates. also calculate the liquid and vapor compositions (mole fractions) at those two conditions.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is init...
Questions in other subjects:



Mathematics, 24.05.2021 19:00






Mathematics, 24.05.2021 19:00

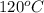 = (120 + 273.15)K = 393.15 K,
= (120 + 273.15)K = 393.15 K, 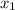 = 0.5 and
= 0.5 and 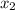 = 0.5
= 0.5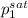 (393.15 K) = 9.2 bar
(393.15 K) = 9.2 bar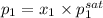
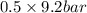
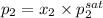
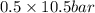
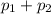
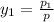
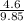
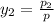
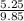
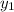 = 0.5,
= 0.5, 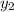 = 0.5
= 0.5 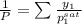
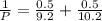
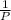 = 0.101966
= 0.101966 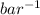
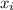 and its formula is as follows.
and its formula is as follows.