

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3


Chemistry, 23.06.2019 06:00, mustafajibawi1
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
What is the percent by mass of oxygen in a gaseous mixture whose molar composition is 0.500 % co2 an...
Questions in other subjects:

English, 19.09.2019 16:40

History, 19.09.2019 16:40

History, 19.09.2019 16:40

Social Studies, 19.09.2019 16:40


English, 19.09.2019 16:40


Biology, 19.09.2019 16:40


Mathematics, 19.09.2019 16:40

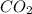 = 0.500 %
= 0.500 %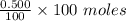 = 0.5 moles
= 0.5 moles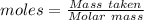
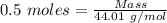
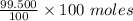 = 99.500 moles
= 99.500 moles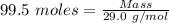
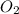 = 21 % of air
= 21 % of air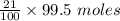 = 20.895 moles
= 20.895 moles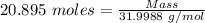
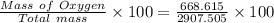 = 23.0 %
= 23.0 %

