
Chemistry, 10.10.2019 05:30 Queenbee2304
Calculate the radius of tantalum (ta) atom, given that ta has a bcc crystal structure, a density of 16.6 g/cm, and an atomic weight of 180.9 g/mol. (avogadro number, 6.023 x 103 atoms/mol).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Calculate the radius of tantalum (ta) atom, given that ta has a bcc crystal structure, a density of...
Questions in other subjects:

Mathematics, 27.09.2019 06:30



Biology, 27.09.2019 06:30


Geography, 27.09.2019 06:30


Mathematics, 27.09.2019 06:30

History, 27.09.2019 06:30

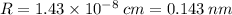
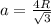
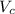 is
is 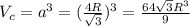
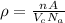
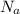 = Avogadro’s number (
= Avogadro’s number (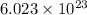 atoms/mol)
atoms/mol)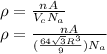
![\rho=\frac{nA}{(\frac{64\sqrt{3}R^3}{9})N_{a}}\\\frac{64\sqrt{3}R^3}{9}=\frac{nA}{\rho N_{a}}\\R^3=\frac{nA}{\rho N_{a}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}} \\R=\sqrt[3]{\frac{nA}{\rho N_{a}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}}}](/tpl/images/0306/0375/e96f7.png)
![R=\sqrt[3]{\frac{2\cdot 180.9}{16.6\cdot 6.023 \times 10^{23}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}}}\\R = 1.43 \times 10^{-8} \:cm = 0.143 \:nm](/tpl/images/0306/0375/377b1.png)



