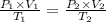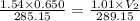Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressure is 1.54 atm and the temperature is 12°c. determine the bubble’s volume upon rising near the top where the pressure is 1.01 atm and 16°c. assume that the number of moles of helium remains constant and that the helium is an ideal gas. (13 pts)
important equations and constants 1 atm = 760 torr = 760 mmhg = 101,325 pa
1ml = 1cm3
pv = nrt
p1v1 = p2v2
v1/t1 = v2/t2
v1/n1 = v2/n2
ptotal = p1 + p2 + p3 + ….
(p1v1)/(n1t1) = (p2v2)/(n2t2)
r = 0.08206 l atm/mol k

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:00, Ezekielcassese
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 06:00, mirzakasumovic8926
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressu...
Questions in other subjects:

Social Studies, 28.09.2019 13:10



English, 28.09.2019 13:10

Mathematics, 28.09.2019 13:10

Spanish, 28.09.2019 13:10

English, 28.09.2019 13:10


English, 28.09.2019 13:10

History, 28.09.2019 13:10