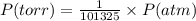Chemistry, 10.10.2019 05:20 hillarytrinh
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, are injected into an evacuated 536 cm3 flask.
a. find the number of moles of oxygen present prior to mixing the gases, assuming the temperature remains constant, and that oxygen is an ideal gas. (5pts)
b. find the number of moles of nitrogen present prior to mixing the gases, assuming the temperature remains constant and that nitrogen is an ideal gas. (5pts)
c. find the total pressure of oxygen and nitrogen in the flask (after the two gases are mixed together.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1
You know the right answer?
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, a...
Questions in other subjects:

Mathematics, 15.11.2019 07:31

Social Studies, 15.11.2019 07:31

English, 15.11.2019 07:31


Mathematics, 15.11.2019 07:31

Mathematics, 15.11.2019 07:31


Mathematics, 15.11.2019 07:31


Mathematics, 15.11.2019 07:31