
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
e5 = -7 x 10-19 j
e4 = -11 x 10-19 j
e3 = -15 x 10-19 j
e2 = -17 x 10-19 j
e1 = -20 x 10-19 j
(this is not an actual atom that exists. according to the bohr model, energies should follow the expression discussed in section 6.2 of silberberg. this exercise prepares you for calculations like questions 6 and 7 of problem set 1.)
(a) if the electron is initially in the n = 4 level, what is the shortest wavelength of radiation that could be emitted? provide your answer to one significant figure: -7 m.
(b) what is the ionization energy (in kj/mol) of the atom in its first excited state? provide your answer to two significant figures: 103 kj/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 06:30, kaitlynk0
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
...
e6 = -2 x 10-19 j
...
Questions in other subjects:



History, 22.04.2021 23:20

Mathematics, 22.04.2021 23:20




Mathematics, 22.04.2021 23:20

Mathematics, 22.04.2021 23:20

Mathematics, 22.04.2021 23:20

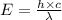
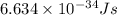
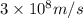
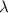 = Wavelength of the radiation
= Wavelength of the radiation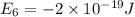
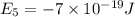
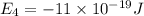
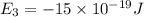
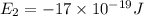
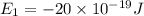
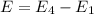
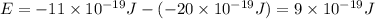
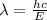
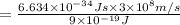
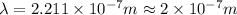
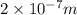 is the shortest wavelength of radiation that could be emitted.
is the shortest wavelength of radiation that could be emitted.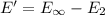
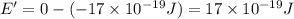
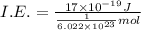
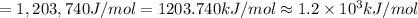
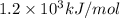 is the ionization energy of the atom in its first excited state.
is the ionization energy of the atom in its first excited state.

