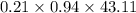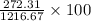Chemistry, 10.10.2019 05:00 cgattis6935
Amixture of a and b is capable of being ignited only if the mole percent of a is 6 %. a mixture containing 9.0 mole% a in b flowing at a rate of 800 kg/h is to be diluted with pure b to reduce a concentration to the lower flammability limit. calculate the required flow rate of b in mol/h and the percent by mass of oz in the product gas. (note: b may be taken to consist of 21 mole% o2 and 79% nz and to have an average molecular weight of 29.0.) ma = 16.04 g/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, salvadorperez26
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3

Chemistry, 21.06.2019 15:40, lexybellx3
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Amixture of a and b is capable of being ignited only if the mole percent of a is 6 %. a mixture cont...
Questions in other subjects:


Mathematics, 03.11.2020 20:50




Mathematics, 03.11.2020 20:50



Arts, 03.11.2020 20:50

History, 03.11.2020 20:50

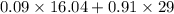
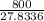
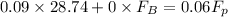
 = 43.11 kmol/hr
= 43.11 kmol/hr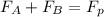
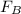 = 43.11 - 28.74
= 43.11 - 28.74
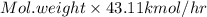

 into the product stream is as follows.
into the product stream is as follows.