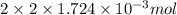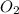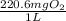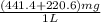Chemistry, 10.10.2019 05:00 geunagillis1
Determine the carbonaceous and nitrogenous oxygen demand in mg/l for a 1 l solution containing 300 mg of a wastewater represented by the formula cn2h602 (n is converted to nh3 in the first step) afterwards calculate the cod of the solution

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 23.06.2019 02:30, ggpro4life3000
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
Determine the carbonaceous and nitrogenous oxygen demand in mg/l for a 1 l solution containing 300 m...
Questions in other subjects:

English, 17.06.2021 22:10

English, 17.06.2021 22:10


Mathematics, 17.06.2021 22:10


Biology, 17.06.2021 22:10




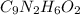 .
.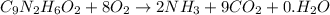
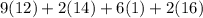
 mg/mol (as 1 g = 1000 mg)
mg/mol (as 1 g = 1000 mg)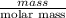
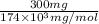
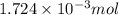
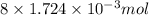
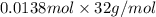
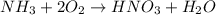
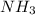 to convert into
to convert into 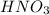 , oxygen required is 2 mol.
, oxygen required is 2 mol.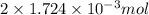 of
of