
Chemistry, 10.10.2019 04:10 emmabarnett817
What is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00 l of 0.0100 m benzoic acid, hc7h5o2? assume that there is no change in volume. the ka for benzoic acid is 6.3 × 10–5. view available hint(s) what is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00 l of 0.0100 m benzoic acid, hc7h5o2? assume that there is no change in volume. the ka for benzoic acid is 6.3 × 10–5. 4.33 3.76 0.439 4.64

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:00, onegirl435
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
You know the right answer?
What is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00...
Questions in other subjects:







Mathematics, 04.08.2019 12:20

Mathematics, 04.08.2019 12:20


History, 04.08.2019 12:20



![[\text{A}^{-}] = \dfrac{\text{0.027 49 mol}}{\text{1 L}} = \text{0.027 49 mol/L }](/tpl/images/0305/8117/b8b4c.png)
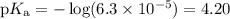
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log\dfrac{[\text{A}^{-}]}{\text{[HA]}}\\\\& = & 4.20 +\log\dfrac{0.02749}{0.0100}\\\\& = & 4.20 + \log2.749 \\& = & 4.20 + 0.4390\\& = & 4.64\\\end{array}\\\text{The pH of the buffer is $\large \boxed{\mathbf{4.64}}$}](/tpl/images/0305/8117/4e2e9.png)


