
Chemistry, 10.10.2019 04:10 princess42044
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2. he pipets 20 ml of this solution into a 100 ml volumetric flask and dilutes to the mark. he then pipets 10 ml of this diluted solution into a 100 ml volumetric flask and dilutes to the mark. he analyzes some of the solution from the final volumetric flask and finds that the iodide ion concentration is 0.574 m. (note: in solution, calcium iodide breaks apart into one ca 2+ ion for every two i - ions, so a solution that is 1.0 m in cai 2 is 2.0 m in i determine the molar concentration of calcium iodide in the original solution.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2...
Questions in other subjects:










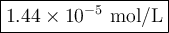
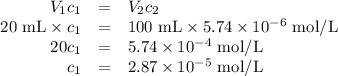
![[\text{Ca}^{2+}] =\dfrac{2.87 \times 10^{-5} \text{ mol I}^{-}}{\text{1 L}} \times \dfrac{\text{1 mol Ca}^{2+} }{\text{2 mol I}^{-}} = \mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}\\\\\text{[Ca$^{2+}$] in the original solution was $\large \boxed{\mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}}$}](/tpl/images/0305/8108/dfb7a.png)


