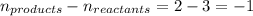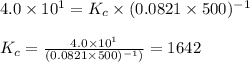The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three gases has partial pressures of 9.10×10−2 atm , 0.174 atm , and 0.24 atm for no, cl2, and nocl, respectively. part apart complete calculate kp for this reaction at 500.0 k. express your answer using two significant figures. kp = 40 previous answers correct part b if the vessel has a volume of 6.00 l , calculate kc at this temperature. express your answer using two significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, anarosa331hotmailcom
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 21.06.2019 23:30, 23gordns
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉mo re accurate estimates can be made with the van der waals equationí‘ťí‘ť=í‘›í‘›í‘›í‘›í‘›í‘›í‘ ‰í‘‰â’푛푛푟푟â’푞푞푛푛2í‘ ‰í‘‰2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three...
Questions in other subjects:



Mathematics, 09.01.2021 09:20

Mathematics, 09.01.2021 09:20

Mathematics, 09.01.2021 09:20

Mathematics, 09.01.2021 09:20

Mathematics, 09.01.2021 09:20

Social Studies, 09.01.2021 09:20


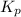 for the given reaction is
for the given reaction is 
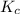 for the given reaction is 1642.
for the given reaction is 1642.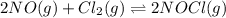
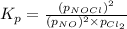
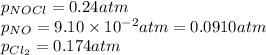
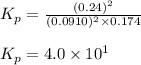
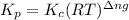
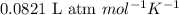
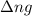 = change in number of moles of gas particles =
= change in number of moles of gas particles =