
For the following reaction, kc = 15 at 700 k. 2 no(g) + cl2(g) ⇄ 2 nocl(g) if we have [no] = 0.15 m, [cl2] = 0.15 m, [nocl] = 0.40 m at 700 k, what will happen? group of answer choices the equilibrium will not shift. the equilibrium will shift to the left, but will use up only part of the nocl. the equilibrium will shift to the right, but will use up only part of the no and cl2. the equilibrium will shift to the right until all the reactants are used up. the equilibrium will shift to the left until all the nocl is used up.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2

Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
For the following reaction, kc = 15 at 700 k. 2 no(g) + cl2(g) ⇄ 2 nocl(g) if we have [no] = 0.15 m,...
Questions in other subjects:

Mathematics, 21.01.2021 07:10

English, 21.01.2021 07:10


Mathematics, 21.01.2021 07:10

Mathematics, 21.01.2021 07:10

Mathematics, 21.01.2021 07:10

History, 21.01.2021 07:10

Mathematics, 21.01.2021 07:10

Arts, 21.01.2021 07:10

Mathematics, 21.01.2021 07:10

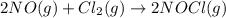
![Q=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0305/1218/afbe9.png)
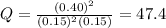
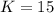
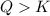 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.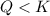 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.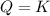 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

