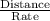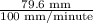In a gas chromatography experiment, a mixture of two compounds (a and b) was separated for analysis. a chart recorder that fed paper at a constant rate of 100 mm. per minute was used to record the chromatogram, and a mark was made on the chart at the time the sample was injected. the first peak on the chromatogram corresponds to compound a, and that peak was observed at a distance of 5.83 cm from the sample injection point, and it had a height of 3.52 cm and a width at half-height of 1.25 cm. the peak corresponding to b was observed at a distance of 7.96 cm from the injection point, and it had a height of 3.34 cm and a width at half-height of 1.06 cm. what was the retention time for compound b (in seconds)? (enter your answer as a number only.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, carog24
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 01:30, AptAlbatross
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

You know the right answer?
In a gas chromatography experiment, a mixture of two compounds (a and b) was separated for analysis....
Questions in other subjects:

Social Studies, 16.07.2019 23:30

Mathematics, 16.07.2019 23:30

Social Studies, 16.07.2019 23:30



History, 16.07.2019 23:30

Social Studies, 16.07.2019 23:30

History, 16.07.2019 23:30

English, 16.07.2019 23:30

Health, 16.07.2019 23:30