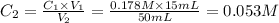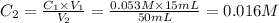Achemist places 2.5316 g of na 2so 4 in a 100 ml volumetric flask and adds water to the mark. she then pipets 15 ml of the resulting solution into a 50 ml volumetric flask and adds water to the mark and mixes to make a solution. she then pipets 15 ml of this new solution into a 50 ml volumetric flask and dilutes to the mark. determine the molar concentration of sodium sulfate in the most dilute solution prepared.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
Achemist places 2.5316 g of na 2so 4 in a 100 ml volumetric flask and adds water to the mark. she th...
Questions in other subjects:

Mathematics, 06.04.2021 22:10


English, 06.04.2021 22:10






Mathematics, 06.04.2021 22:10

![[Na_{2}SO_{4}]=\frac{moles(Na_{2}SO_{4})}{liters(solution)} =\frac{mass((Na_{2}SO_{4}))}{molarmass(moles(Na_{2}SO_{4}) \times 0.100L)} =\frac{2.5316g}{142g/mol\times 0.100L } =0.178M](/tpl/images/0304/7678/6942e.png)