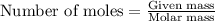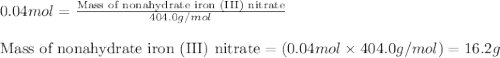In reality, a hydrate of iron(iii) nitrate had to be used, not the anhydrous salt. as you may guess, some of the hydrate’s mass is water, and some is iron(iii) nitrate. how many grams of fe(no3)3•9h2o needed to be dissolved in water to make 2 l of 0.0020 m fe(no3)3? molecular weight of the nonahydrate is 404.0 g/mol. hint: try to set up an equation using x, and solving it. assume that the density of your solution is 1.000 g/ml.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
In reality, a hydrate of iron(iii) nitrate had to be used, not the anhydrous salt. as you may guess,...
Questions in other subjects:


English, 19.03.2020 02:29







Mathematics, 19.03.2020 02:29

Computers and Technology, 19.03.2020 02:29

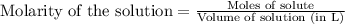
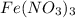 = 0.0020 M
= 0.0020 M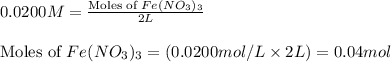
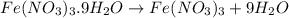
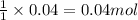 of hydrated iron (III) nitrate
of hydrated iron (III) nitrate