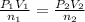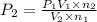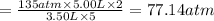Chemistry, 09.10.2019 18:00 carolelai08
Imagine that you have a 5.00 l gas tank and a 3.50 l gas tank. you need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. if you fill the larger tank with oxygen to a pressure of 135 atm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? assume ideal behavior for all gases. express your answer with the appropriate units. view available hint(s) pp = nothingnothing submit provide feedback next

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
Imagine that you have a 5.00 l gas tank and a 3.50 l gas tank. you need to fill one tank with oxygen...
Questions in other subjects:

Mathematics, 28.01.2020 08:31



Physics, 28.01.2020 08:31



Mathematics, 28.01.2020 08:31


Mathematics, 28.01.2020 08:31

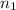
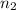
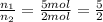
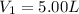
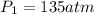
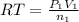 ..[1]
..[1]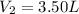
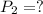
 ..[2]
..[2]