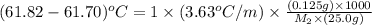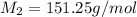Chemistry, 09.10.2019 18:00 Derienw6586
Benzyl acetate is one of the active components of oil of jasmine. if 0.125 g of the compound is added to 25.0g of chloroform (chcl 3 ), the boiling point of the solution is 61.82°c. what is the molar mass of benzyl acetate. (chloroform: kb = 3.63 °c/m; tb° 61.70°c)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, morrisjillian23
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2


Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Benzyl acetate is one of the active components of oil of jasmine. if 0.125 g of the compound is adde...
Questions in other subjects:

Physics, 04.05.2021 06:10

English, 04.05.2021 06:10

Physics, 04.05.2021 06:10


Mathematics, 04.05.2021 06:10

Mathematics, 04.05.2021 06:10

Mathematics, 04.05.2021 06:10

Mathematics, 04.05.2021 06:10

Mathematics, 04.05.2021 06:10

Biology, 04.05.2021 06:10

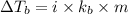
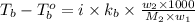
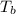 = boiling point of solution =
= boiling point of solution = 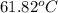
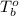 = boiling point of chloroform =
= boiling point of chloroform = 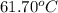
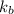 = boiling point constant of chloroform =
= boiling point constant of chloroform = 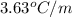
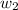 = mass of solute (Benzyl acetate) = 0.125 g
= mass of solute (Benzyl acetate) = 0.125 g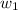 = mass of solvent (chloroform) = 25.0 g
= mass of solvent (chloroform) = 25.0 g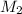 = molar mass of solute (Benzyl acetate) = ?
= molar mass of solute (Benzyl acetate) = ?