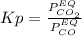Chemistry, 09.10.2019 05:30 minnahelhoor
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s) kp = 20 at 873 k if a reaction vessel at equilibrium contains solid ni, solid nio, 400 mm hg of co2, and 20 mm hg of co, doubling the amount of co(g) present will lead to the production of more solid nickel at 873 k. group of answer choices

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1


Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s)...
Questions in other subjects:

Mathematics, 17.12.2021 03:40




Mathematics, 17.12.2021 03:40





History, 17.12.2021 03:40