
Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 42 cm3. if the combustion of this mixture releases 927 j of energy, to what volume (in l) will the gases expand against a constant pressure of 656 torr if all the energy of combustion is converted into work to push back the piston?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 42...
Questions in other subjects:




English, 28.07.2019 05:30


Physics, 28.07.2019 05:30


Mathematics, 28.07.2019 05:30


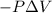
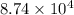 Pa (as 1 torr = 133.3 Pa)
Pa (as 1 torr = 133.3 Pa)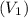 = 42
= 42 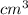
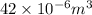 (as 1 m = 100 cm)
(as 1 m = 100 cm)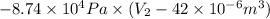
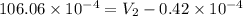
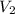 =
= 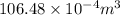
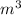 = 1000 L)
= 1000 L)

