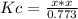Chemistry, 09.10.2019 05:00 katiedurden02
Phosgene is a potent chemical warfare agent that is now outlawed by international agreement. it decomposes by the reaction: cocl2 (g) ⇋ co (g) + cl2 (g) kc = 7.5 x 10-5 (at 362°c) calculate the concentration of co when 7.73 mol of phosgene decomposes and reaches equilibrium in a 10.0 liter flask. multiply your answer by 103 and enter that number to 2 decimal places. hint: look at sample problem 17.9 in the 8th ed of silberberg. write a kc expression. calculate the initial concentration of phosgene. do an ice chart. find the concentration of co.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 07:00, phancharamachasm
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
Phosgene is a potent chemical warfare agent that is now outlawed by international agreement. it deco...
Questions in other subjects:



Mathematics, 02.02.2022 23:20


English, 02.02.2022 23:20




Mathematics, 02.02.2022 23:30

![Kc = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0302/6261/d3f86.png)