
Chemistry, 09.10.2019 03:30 fancycar14
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed to oxygen. the reaction is 2b5h9(l) + 12o2(g) ⟶ 5b2o3(s) + 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formation of b5h9 is 73.2 kj/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, dontcareanyonemo
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Chemistry, 21.06.2019 17:00, porkhappycom
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed...
Questions in other subjects:


Social Studies, 16.10.2020 09:01

English, 16.10.2020 09:01



Chemistry, 16.10.2020 09:01

English, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01


Mathematics, 16.10.2020 09:01

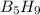 is -71.92 kJ
is -71.92 kJ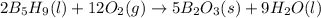
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0302/4270/72c39.png)
![\Delta H^o_{rxn}=[(5\times \Delta H^o_f_{(B_2O_3(s))})+(9\times \Delta H^o_f_{(H_2O(l))})]-[(2\times \Delta H^o_f_{(B_5H_9(l))})+(12\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0302/4270/e310e.png)
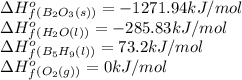
![\Delta H^o_{rxn}=[(5\times (1271.94))+(9\times (-285.83))]-[(2\times (73.2))+(12\times (0))]\\\\\Delta H^o_{rxn}=-9078.57kJ](/tpl/images/0302/4270/015ae.png)
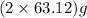 of
of 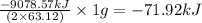 of energy.
of energy.

