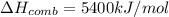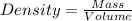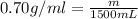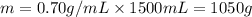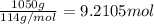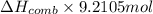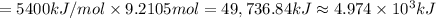The automobile fuel called e85 consists of 85 % ethanol and 15 % gasoline. e85 can be used in so-called "flex-fuel" vehicles (ffvs), which can use gasoline, ethanol, or a mix as fuels. assume that gasoline consists of a mixture of octanes (different isomers of c8h18), that the average heat of combustion of c8h18(l) is 5400 kj/mol, and that gasoline has an average density of 0.70 g/ml. the density of ethanol is 0.79 g/ml. by using the information given, calculate the energy produced by combustion of 1.5 l of gasoline.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
The automobile fuel called e85 consists of 85 % ethanol and 15 % gasoline. e85 can be used in so-cal...
Questions in other subjects:

Biology, 02.08.2019 15:40

History, 02.08.2019 15:40


Biology, 02.08.2019 15:40




Social Studies, 02.08.2019 15:40


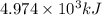 of energy is produced by combustion of 1.5 L of gasoline.
of energy is produced by combustion of 1.5 L of gasoline.