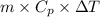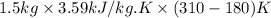Chemistry, 09.10.2019 03:10 beccahaileyowzryu
Cryogenics has the potential to be useful in a variety of fields, including medicine. suppose you have engineered a method to successfully deep-freeze and thaw human organs using liquid nitrogen without any freezing damage to the cells and tissue structure. how much heat must be removed from a liver (1.5 kg) to drop its temperature from 310 k to 180 k and freeze the tissue? for liquids and solids, heat capacity at constant pressure, cp, is approximately equal to heat capacity at constant volume, cv.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 07:30, jessicawelch25
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2

Chemistry, 23.06.2019 10:00, darajeanty2004p7cu4m
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
Cryogenics has the potential to be useful in a variety of fields, including medicine. suppose you ha...
Questions in other subjects:

Social Studies, 06.11.2020 06:40


Computers and Technology, 06.11.2020 06:40


Computers and Technology, 06.11.2020 06:40


Physics, 06.11.2020 06:40



Mathematics, 06.11.2020 06:40

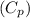 is 3.59 kJ
is 3.59 kJ 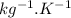
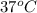 = 310 K
= 310 K