
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced. caco3(s)+2hcl(aq)⟶cacl2(aq)+h2o(l)+ co2(g) how many grams of calcium chloride will be produced when 31.0 g of calcium carbonate is combined with 13.0 g of hydrochloric acid?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3


Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3
You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions in other subjects:

Biology, 07.11.2020 08:20


English, 07.11.2020 08:20


Mathematics, 07.11.2020 08:20



Social Studies, 07.11.2020 08:20

English, 07.11.2020 08:20



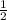 mole of calcium chloride
mole of calcium chloride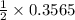 mole of calcium chloride
mole of calcium chloride

