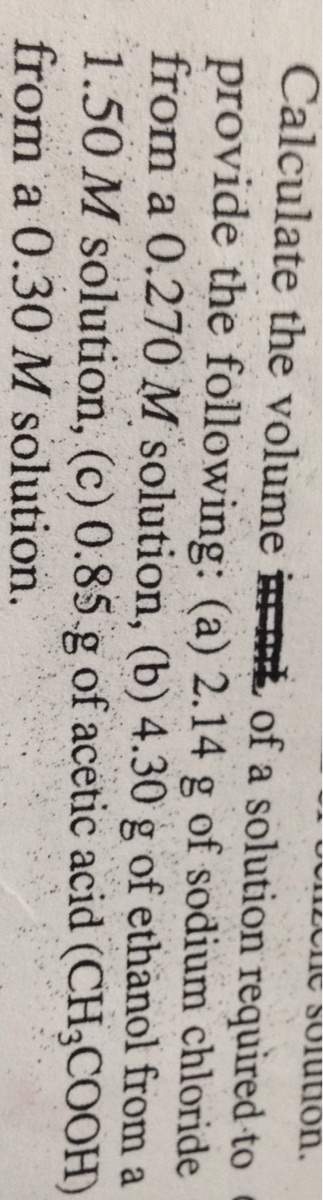Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, steven2996
What can be the use of smoke transformed into liquid?
Answers: 1

Chemistry, 21.06.2019 16:00, robert7248
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 21:50, isabel81ie
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l. s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Calculate the volume a solution required toof provide the following: (a) 2.14 g of sodium chloridef...
Questions in other subjects:




English, 17.12.2021 01:00

Mathematics, 17.12.2021 01:00


History, 17.12.2021 01:00

Health, 17.12.2021 01:00


Mathematics, 17.12.2021 01:00