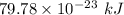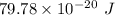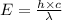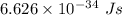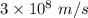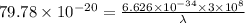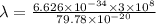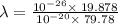Chemistry, 08.10.2019 17:30 newtonthenewt
The work function of an element is the energy required to remove an electron from the surface of the solid. the work function for rhodium is 480.5 kj/mol (that is, it takes 480.5 kj of energy to remove 1 mole of electrons from 1 mole of rh atoms on the surface of rh metal). what is the maximum wavelength of light that can remove an electron from an atom in rhodium metal?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 09:00, yogibear5806
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
The work function of an element is the energy required to remove an electron from the surface of the...
Questions in other subjects:


History, 03.08.2019 07:30

Biology, 03.08.2019 07:30




Mathematics, 03.08.2019 07:30



History, 03.08.2019 07:30

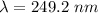
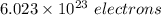
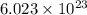 electrons can be removed by applying of 480.5 kJ of energy.
electrons can be removed by applying of 480.5 kJ of energy.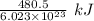 of energy.
of energy.