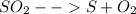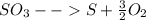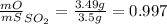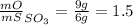Chemistry, 08.10.2019 05:30 aboatright7410
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decomposed the sulfur dioxide produced 3.49g oxygen and 3.50g sulfur, while the sulfur trioxide produced 9.00g oxygen and 6.00g sulfur.
a) calculate the mass of oxygen per gram of sulfur for sulfur dioxide.
b) calculate the mass of oxygen per gram of sulfur for sulfur trioxide.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, hailiemanuel3461
Select the correct answer from each drop-down menu. daniel and sanya are scientists. daniel is studying whether the increasing frequency of tropical storms is affecting coastal erosion. sanya is investigating whether the discharge from industrial plants has any impact on the ph concentration of freshwater swamps in the surrounding area. which fields of science are daniel’s and sanya’s studies most closely related to? daniel’s field of study is related to science, and sanya’s field of study is related to .
Answers: 3

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decompose...
Questions in other subjects:

Mathematics, 08.11.2019 04:31


English, 08.11.2019 04:31

Mathematics, 08.11.2019 04:31

Health, 08.11.2019 04:31



Chemistry, 08.11.2019 04:31