
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to remove h2s is to treat the water with chlorine, in which case the following reaction occurs: h2s(aq)+cl2(aq)→s(s)+2h+(aq)+2cl−(a q) the rate of this reaction is first order in each reactant. the rate constant for the disappearance of h2s at 28 ∘c is 3.5×10−2 m−1s−1.if at a given time the concentration of h2s is 2.0×10-4 m and that of cl2 is 2.8×10-2 m , what is the rate of formation of cl?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to r...
Questions in other subjects:


History, 26.06.2019 08:20

Mathematics, 26.06.2019 08:20

Biology, 26.06.2019 08:30


Chemistry, 26.06.2019 08:30


Physics, 26.06.2019 08:30

English, 26.06.2019 08:30

History, 26.06.2019 08:30

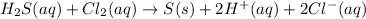
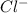 will be twice the rate of disappearance of
will be twice the rate of disappearance of 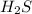 .
.![k[H_{2}S][Cl_{2}]](/tpl/images/0299/3822/2cbd6.png)
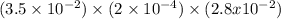
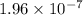 M/s
M/s
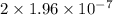
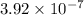 M/s
M/s

