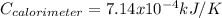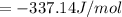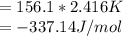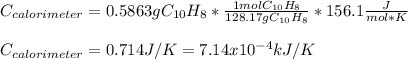When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter constant, a temperature rise of 2.862 k was measured near 298k. under similar conditions, a temperature rise of 2.416 k was measured when a 0.5863 g naphthalene sample was burned. determine the calorimeter constant (in units of kj/k) and the standard enthalpy of combustion for naphthalene at 298k. if we make the assumption that ∆h ≈∆u, is the ∆chº value obtained from this experim

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 08:00, kathrynpuppies201716
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1

Chemistry, 23.06.2019 22:30, tabocampos1414
Acompound consisting of b, n, and h undergoes elemental analysis. the % composition by mass is found to be 40.28% b, 52.20% n, and 7.53% h. what is the empirical formula for this molecule? enter in the empirical formula in the form bxnyhz
Answers: 1
You know the right answer?
When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter co...
Questions in other subjects:



History, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01



Mathematics, 13.10.2020 02:01