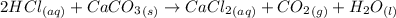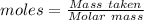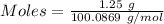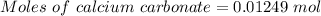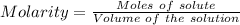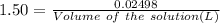Chemistry, 08.10.2019 05:10 pressure772
Calcium carbonate is added to separate solutions of hydrochloric acid and ethanoic acid of the same concentration. state one similarity and one difference in the observations you could make.
(i) write an equation for the reaction between hydrochloric acid and calcium carbonate.
(ii) determine the volume of 1.50 mol/dm–3 hydrochloric acid that would react with exactly 1.25 g of calcium carbonate.
(iii) calculate the volume of carbon dioxide, measured at 273 k and 1.01×105 pa, which would be produced when 1.25 g of calcium carbonate reacts completely with the hydrochloric acid.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
You know the right answer?
Calcium carbonate is added to separate solutions of hydrochloric acid and ethanoic acid of the same...
Questions in other subjects:

Advanced Placement (AP), 30.11.2020 20:50

Mathematics, 30.11.2020 20:50





Mathematics, 30.11.2020 20:50

Mathematics, 30.11.2020 20:50

Mathematics, 30.11.2020 20:50

Geography, 30.11.2020 20:50