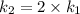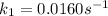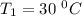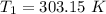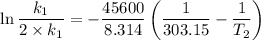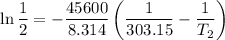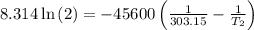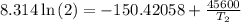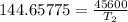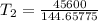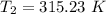Chemistry, 08.10.2019 03:20 jonlandis6
The arrhenius equation shows the relationship between the rate constant k and the temperature t in kelvins and is typically written as k=ae−ea/rt where r is the gas constant (8.314 j/mol⋅k), a is a constant called the frequency factor, and ea is the activation energy for the reaction. however, a more practical form of this equation is lnk2k1=ear(1t1−1t2) which is mathematically equivalent to lnk1k2=ear(1t2−1t1) where k1 and k2 are the rate constants for a single reaction at two different absolute temperatures (t1 and t2). part a the activation energy of a certain reaction is 45.6 kj/mol . at 30 ∘c , the rate constant is 0.0160s−1 . at what temperature in degrees celsius would this reaction go twice as fast

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 20:30, moneykey
Hannah is writing a report on how albedo affects the global climate. she’s proofreading her passage for any factual errors. which sentence must hannah correct before submitting her report? earth receives energy from the sun. this energy drives many of the processes on earth, including its climate. some part of this energy is reflected by earth’s surface. we use the term albedo to describe the reflected energy. albedo of an object is the ratio of the reflected radiation to the total radiation reaching the object. a value of 0 means no energy is absorbed by the object, whereas a value of 1 means that all of the energy is absorbed. in this way, the albedo of an object can influence earth’s atmospheric temperature.
Answers: 1

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3
You know the right answer?
The arrhenius equation shows the relationship between the rate constant k and the temperature t in k...
Questions in other subjects:






Mathematics, 26.03.2020 01:55




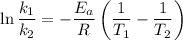
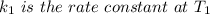
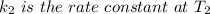
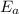 is the activation energy
is the activation energy