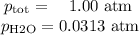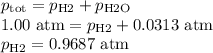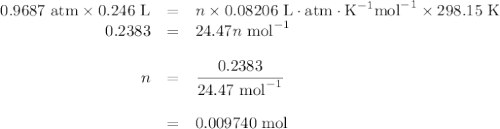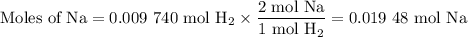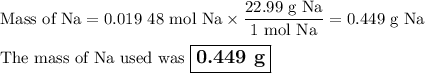Chemistry, 08.10.2019 02:10 lazymarshmallow7
Apiece of sodium metal reacts completely with water as follows: 2na(s) + 2h2o(l) ⟶ 2naoh(aq) + h2(g) the hydrogen gas generated is collected over water at 25.0°c. the volume of the gas is 246 ml measured at 1.00 atm. calculate the number of grams of sodium used in the reaction. (vapor pressure of water at 25°c = 0.0313 atm.)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1


You know the right answer?
Apiece of sodium metal reacts completely with water as follows: 2na(s) + 2h2o(l) ⟶ 2naoh(aq) + h2(g...
Questions in other subjects:

Mathematics, 19.08.2021 01:20


Mathematics, 19.08.2021 01:20






Mathematics, 19.08.2021 01:20

Biology, 19.08.2021 01:20