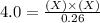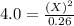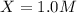Chemistry, 08.10.2019 01:00 hamadehassan
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l container and allowed to come to equilibrium, and the equilibrium concentration of pcl5(g) is 0.26 m, what is the equilibrium concentration of pcl3?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, penelopymorales24
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


You know the right answer?
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l...
Questions in other subjects:


Mathematics, 28.12.2020 20:50


Mathematics, 28.12.2020 21:00



Biology, 28.12.2020 21:00



Social Studies, 28.12.2020 21:00

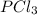 is, 1.0 M
is, 1.0 M = 0.26 M
= 0.26 M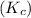 = 4.0
= 4.0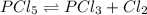
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0298/6790/73fe0.png)
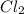 are equal.
are equal.