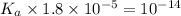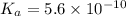Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 12:10, rainbowboy9231
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
Given that at 25.0 ∘c ka for hcn is 4.9×10−10 and kb for nh3 is 1.8×10−5, calculate kb for cn− and k...
Questions in other subjects:



Mathematics, 27.01.2020 03:31



Mathematics, 27.01.2020 03:31


Mathematics, 27.01.2020 03:31


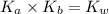
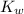 is the dissociation constant of water.
is the dissociation constant of water.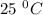 ,
, 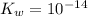
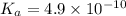
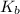 for CN⁻ can be calculated as:
for CN⁻ can be calculated as:
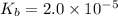
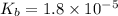
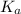 for
for 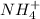 can be calculated as:
can be calculated as: