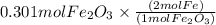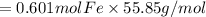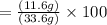Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1


You know the right answer?
Combining 0.301 mol fe2o3
with excess carbon produced 11.6 g fe.
fe2o3+3c⟶2fe+3co<...
with excess carbon produced 11.6 g fe.
fe2o3+3c⟶2fe+3co<...
Questions in other subjects:








English, 31.07.2019 21:40


Health, 31.07.2019 21:40

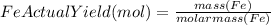
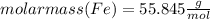
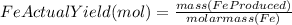
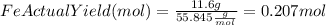
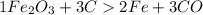
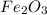 to moles Fe and moles Fe to mass Fe
to moles Fe and moles Fe to mass Fe 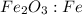 is 1 : 2
is 1 : 2