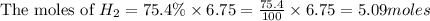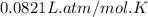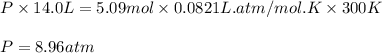Chemistry, 07.10.2019 23:00 brandytyler317fries
Consider 2 al + 6 hcl → 2 alcl3 + 3 h2 , the reaction of al with hcl to produce hydrogen gas. what is the pressure of h2 if the hydrogen gas collected occupies 14.0 l at 300.k and was produced upon reaction of 4.50 moles of al and excess hcl in a process that has a 75.4 percent yield?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2


Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Consider 2 al + 6 hcl → 2 alcl3 + 3 h2 , the reaction of al with hcl to produce hydrogen gas. what i...
Questions in other subjects:





Mathematics, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01




Mathematics, 11.10.2020 01:01

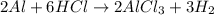
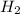 gas
gas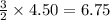 moles of
moles of