
Chemistry, 07.10.2019 21:30 Kaitneedshelps
Apiece of aluminum foil 1.00cm square and 0.590mm thick is allowed to react with bromine to form aluminum bromide. part a how many moles of aluminum were used? (the density of aluminum is 2.699 g/cm^3 part b how many grams of aluminum bromide form, assuming that the aluminum reacts completely?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Apiece of aluminum foil 1.00cm square and 0.590mm thick is allowed to react with bromine to form alu...
Questions in other subjects:

Mathematics, 28.11.2020 02:50

Mathematics, 28.11.2020 02:50




Computers and Technology, 28.11.2020 02:50






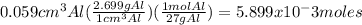
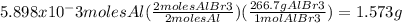 of Al
of Al

