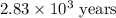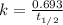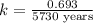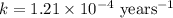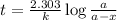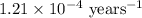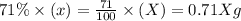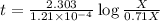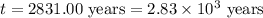Chemistry, 07.10.2019 17:30 animationfusion
Carbon-14 is a radioactive isotope that decays according to first-order kinetics in a process that has a half-life of 5730 years. if a sample containing carbon-14 now has 71% of its original concentration of carbon-14, how much time has passed in years? 4.09 ~ 103 years 5.73 x 103 years 2.38 x 103 years 2.83 x 103 years 3.52 * 104 years

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 03:00, winterblanco
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
Carbon-14 is a radioactive isotope that decays according to first-order kinetics in a process that h...
Questions in other subjects:




History, 05.05.2020 14:28

History, 05.05.2020 14:28

Mathematics, 05.05.2020 14:28

Mathematics, 05.05.2020 14:28

Mathematics, 05.05.2020 14:28