
Chemistry, 07.10.2019 00:20 alisonlebron15
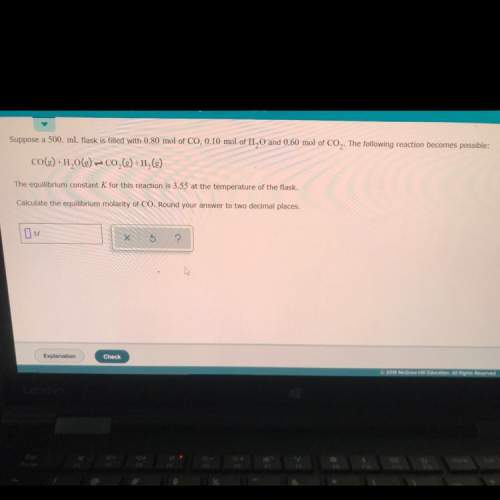
Suppose a 500. ml flask is filled with 0.80 mol of co,0.10 mol of h20 and 0.60 mol of co2. the following reaction becomes possible:
co(g) +h2o(g) + co2(g) +h2(g)
the equilibrium constant k for this reaction is 3.55 at the temperature of the flask.
calculate the equilibrium molarity of co. round your answer to two decimal places.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 03:10, mani1682
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 11:00, lildestinyquintana
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
Suppose a 500. ml flask is filled with 0.80 mol of co,0.10 mol of h20 and 0.60 mol of co2. the follo...
Questions in other subjects:

Mathematics, 23.12.2020 06:30

Mathematics, 23.12.2020 06:30

Mathematics, 23.12.2020 06:30









