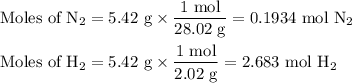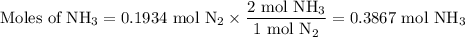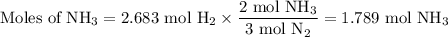Chemistry, 07.10.2019 00:10 ujusdied5176
N2(g) + 3 h2(g) > 2 hn3(g) (blanced)
if 5.42 g of nitrogen are reacted with 5.42 g of hydrogen gas, which of the reactants is the limiting reactant?
molar mass of n2 = 28.02 g/mol
molar mass of h2 = 2.02 g/mol
molar mass of nh3 = 17.04 g/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Kaylinne1181
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

You know the right answer?
N2(g) + 3 h2(g) > 2 hn3(g) (blanced)
if 5.42 g of nitrogen are reacted with 5.42 g o...
if 5.42 g of nitrogen are reacted with 5.42 g o...
Questions in other subjects:



Mathematics, 10.04.2020 16:17



Mathematics, 10.04.2020 16:17

Mathematics, 10.04.2020 16:17