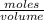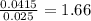Chemistry, 06.10.2019 10:02 Smartpotato9555
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1678 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrating it with the standard solution. the endpoint was reached after the addition of 19.88 ml of the standard solution. what is the concentration of the original sulfuric acid solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standa...
Questions in other subjects:

Mathematics, 22.03.2020 11:52

Mathematics, 22.03.2020 11:53

Computers and Technology, 22.03.2020 11:54


Chemistry, 22.03.2020 11:56

English, 22.03.2020 11:56

English, 22.03.2020 11:59


Mathematics, 22.03.2020 12:09

Mathematics, 22.03.2020 12:11

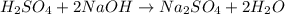
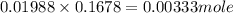
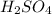 in 10 mL of diluted solution = 1/2 x moles of NaOH
in 10 mL of diluted solution = 1/2 x moles of NaOH
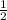 x 0.00333 = 0.00166 mol
x 0.00333 = 0.00166 mol
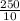 x 0.00166 = 0.0415 mol
x 0.00166 = 0.0415 mol