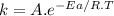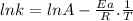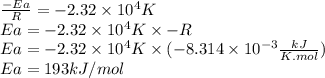Chemistry, 05.10.2019 04:20 Dreynolds1667
For the gas phase isomerization of cis-cyanostyrene, cis-c6h5ch=chc --> ntrans-c6h5ch=chcn the rate constant has been determined at several temperatures. when ln k in s-1 is plotted against the reciprocal of the kelvin temperature, the resulting linear plot has a slope of -2.32×104 k and a y-intercept of 26.7. the activation energy for the gas phase isomerization of cis-cyanostyrene is kj/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 17:30, Anderson0300
With carbon dioxide what phase change take place when the temperature decreases from -40c to -80c at 2 atm
Answers: 2
You know the right answer?
For the gas phase isomerization of cis-cyanostyrene, cis-c6h5ch=chc --> ntrans-c6h5ch=chcn the r...
Questions in other subjects:

Social Studies, 27.08.2019 21:40

History, 27.08.2019 21:40

History, 27.08.2019 21:40

World Languages, 27.08.2019 21:40


Physics, 27.08.2019 21:40

Biology, 27.08.2019 21:40



Mathematics, 27.08.2019 21:40