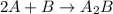Amultistep reaction can only occur as fast as its slowest step. therefore, it is the rate law of the slow step that determines the rate law for the overall reaction. consider the following multistep reaction: a + b → ab (slow) a + ab → a2b (fast)2a + b→ a2b (overall) based on this mechanism, determine the rate law for the overall reaction.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
Amultistep reaction can only occur as fast as its slowest step. therefore, it is the rate law of the...
Questions in other subjects:








Biology, 26.10.2020 20:20

Mathematics, 26.10.2020 20:20

![Rate=k[A][B]](/tpl/images/0288/1503/27e48.png)
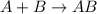 (slow)
(slow)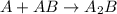 (fast)
(fast)