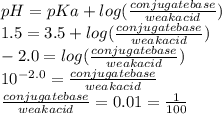Chemistry, 05.10.2019 04:10 meababy2009ow9ewa
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question. aspirin is a weak acid with a pka of 3.5 that is absorbed more effectively in the stomach than the small intestine. the ph of your stomach is around 1.5 and the ph of your small intestine is approximately 6.0. is aspirin absorbed more readily when it is protonated or deprotonated? what is the approximate ratio of conjugate base to acid when it is absorbed more readily?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, leilanimontes714
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question...
Questions in other subjects:

Mathematics, 16.04.2020 01:47


Mathematics, 16.04.2020 01:47


Physics, 16.04.2020 01:48

History, 16.04.2020 01:48