
Chemistry, 06.10.2019 10:00 hannahmorgret7811
Consider the following reaction between mercury(ii) chloride and oxalate ion.
2 hgcl2(aq) + c2o42-(aq) 2 cl -(aq) + 2 co2(g) + hg2cl2(s)
the initial rate of this reaction was determined for several concentrations of hgcl2 and c2o42-, and the following rate data were obtained for the rate of disappearance of c2o42-.
experiment [hgcl2] (m) [c2o42-] (m) rate (m/s)
1 0.164 0.15 3.2x10^-5
2 0.164 0.45 2.9x10^-4
3 0.082 0.45 1.4x10^-4
4 0.246 0.15 4.8x10^-5
what is the rate law for this reaction?
(a) -k[hgcl2][c2o4-2]2-
(b) -k[hgcl2]2[c2o4-2]
(c) -k[hgcl2]2[c2o4-2]1/2

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 05:00, skylarjohnson2683
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
Consider the following reaction between mercury(ii) chloride and oxalate ion.
2 hgcl2(aq...
2 hgcl2(aq...
Questions in other subjects:




English, 17.10.2019 14:10

Mathematics, 17.10.2019 14:10

Social Studies, 17.10.2019 14:10

Biology, 17.10.2019 14:10



Biology, 17.10.2019 14:10

![\text{Rate}=k[HgCl_2][C_2O_4^{2-}]^2](/tpl/images/0293/1267/42a62.png)
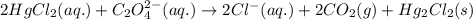
![\text{Rate}=k[HgCl_2]^a[C_2O_4^{2-}]^b](/tpl/images/0293/1267/af610.png)
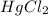
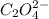
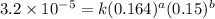 ....(1)
....(1) ....(2)
....(2) ....(3)
....(3)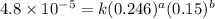 ....(4)
....(4) 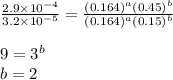

![\text{Rate}=k[HgCl_2]^1[C_2O_4^{2-}]^2](/tpl/images/0293/1267/956be.png)


