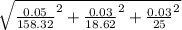Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret is filled with a standardized solution of 158.32 ± 0.05 mm naoh. the initial volume is recorded as 0.14 ml. 25.00 ml of the unknown acid solution are pipetted into an erlenmeyer flask and the solution is titrated to a phenolphthalein endpoint. the final buret reading is 18.76 ml. assuming that the error in each volumetric measurement (buret and pipet) is ±0.03 ml, calculate the concentration of the acid (mm) and use propagation of error to estimate its uncertainty.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret...
Questions in other subjects:

English, 16.12.2020 22:50

Mathematics, 16.12.2020 22:50

Mathematics, 16.12.2020 22:50


Mathematics, 16.12.2020 22:50


Mathematics, 16.12.2020 22:50